Competition tends to bring about a better product or service, at a lower price, than does monopoly. This is a basic premise held by virtually all economists, disputed by pretty much no one in the profession. The entire antitrust edifice of the American system is built upon this foundational aspect of the dismal science.
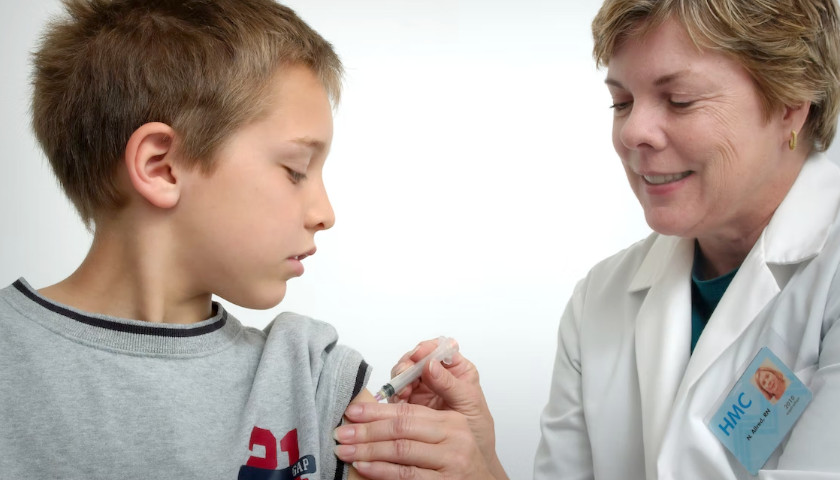
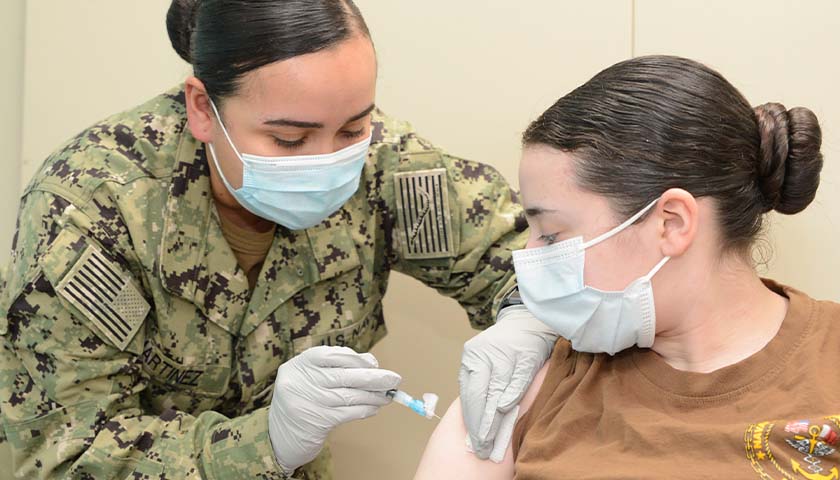
And yet when push comes to shove, our society jettisons this insight, at least when it comes to assuring the quality of our food and drugs.
The Food and Drug Administration is a monopoly agency entrusted with this task. Its word is final concerning such matters. No competition is allowed. If a private agency set itself up as an alternative, it would first be subjected to raucous laughter, and then its creators jailed.
The FDA is a licensing agency. If it does not approve of a food or drug, it is illegal to offer it for sale. What is the non-monopolistic alternative to this sad state of affairs? This is called certification. How, pray tell, does this work? It is simple. Different firms set themselves up as evaluators of the quality of food and drugs, and each of them subjects these products to their examinations. They certify some as approved, and list others as not approved.
Read More